Posted on January 23, 2019 by Faith M. Smith
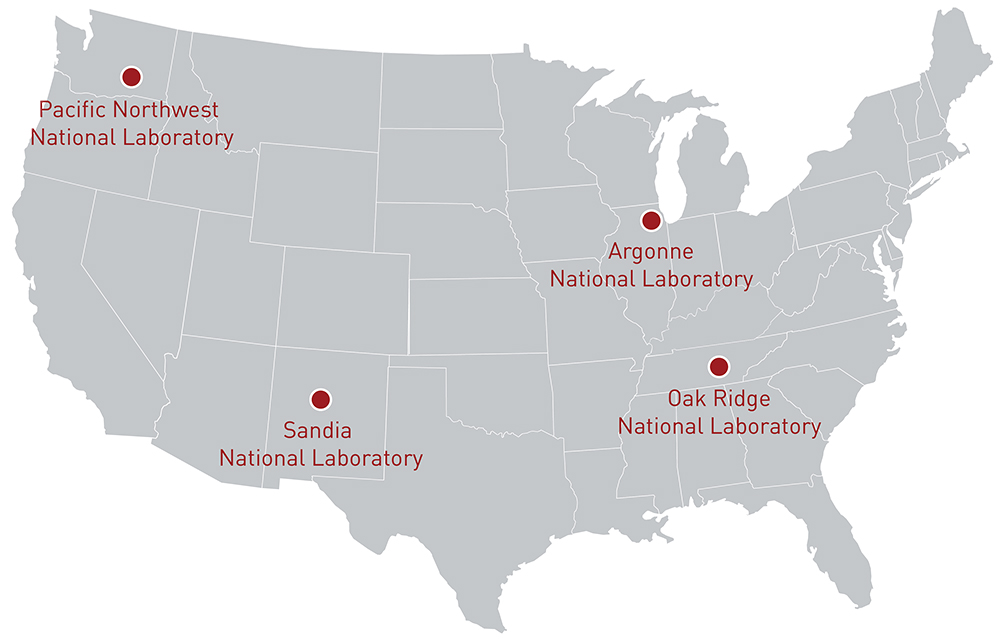
Energy storage as a technology has been around for almost a hundred years in the United States and Europe through pumped hydroelectric storage.2 Modern energy storage as we know it began in 1978 at Sandia National Lab through a program called “Batteries for Specific Solar Applications,” which focused on developing batteries along with other renewables.3 This program began shortly after the formal creation of the Department of Energy and was expanded quickly with a focus on batteries for electric vehicles. Over time, this program, now known as the Energy Storage Systems Program (ESSP) grew to include additional energy storage technologies such as flywheels and compressed air energy storage. While the ESSP initially began at Sandia National Lab, cross-collaborative research from materials science to demonstrations of energy storage technologies continues at Argonne National Lab, Oak Ridge National Lab, and Pacific Northwest National Lab. Research from these labs have continued to develop in the private sector, alongside separate private research and will continue to evolve over time.
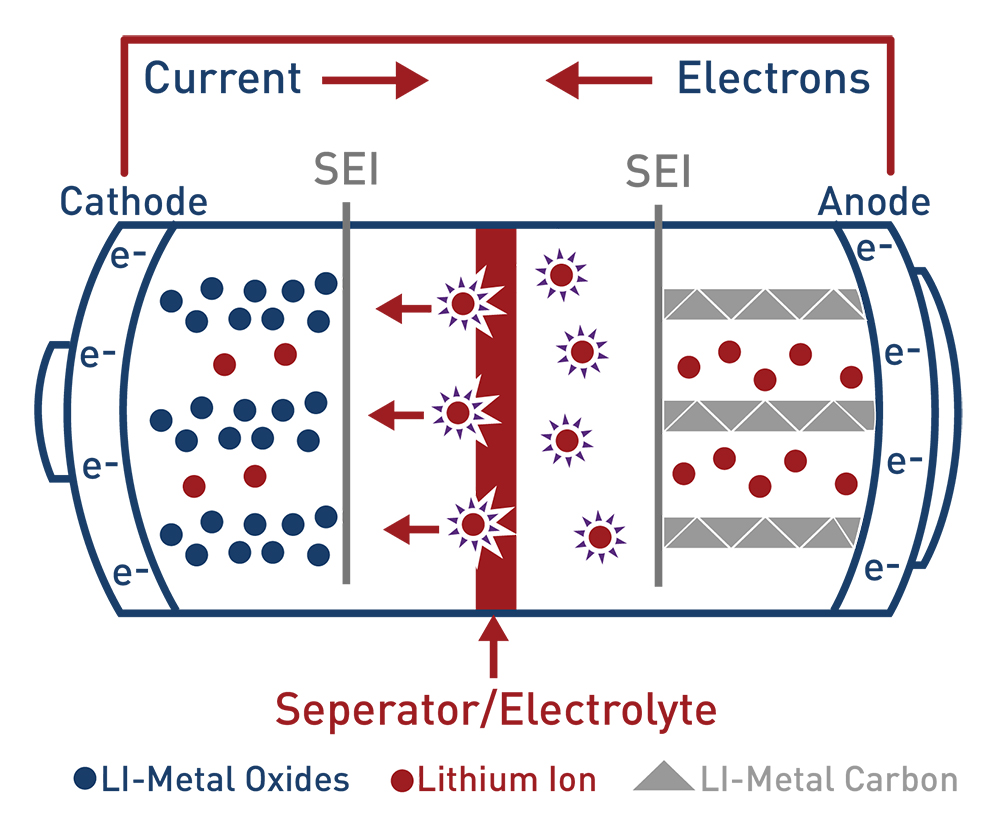
Lithium Ion Batteries
Lithium-ion batteries range in chemistry composition, but all lithium-ion batteries transfer lithium-ions between their cathode and anode, where the cathode is usually a metal oxide and the most common anode is made up of graphite. Lithium-ion batteries can have a liquid electrolyte or a solid state electrolyte. Lithium-ion batteries have a lithium polymer where the electrodes are bonded together by a porous polymer matrix. This means the battery itself has a specific chemistry that varies, changing the battery’s capabilities. Depending on chemistry differences, there are benefits and concerns with each type as well as some being more successful than others. The benefits of lithium ion include: high energy density, less expensive, have long lifetime cycles, are rechargeable, have low maintenance, and have high rate discharge capability. Lithium-ion batteries have been successfully deployed in electric vehicles, mobile phones, and laptops. There is growth in research, design, and manufacturing for large scale projects for use in grid scale storage applications.
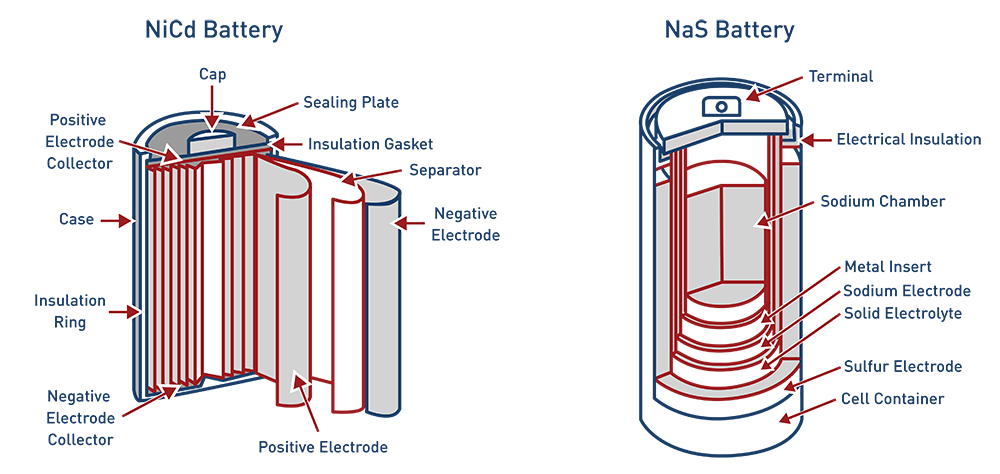
Solid State Batteries have solid electrodes, cathodes, and anodes regardless of their chemistry. Basic chemistry varies in the types of solid state batteries, with three being very common: lithium-ion, nickel-cadmium, and sodium-sulfur. In addition to solid state lithium-ion batteries, other solid state batteries include those with the chemical compositions of nickel-cadmium and sodium-sulfur. Nickel-cadmium batteries are considered a traditional battery, meaning they remain a valid option to provide a long and reliable life cycle even when energy density and cost are not as great as lithium-ion. Nickel-cadmium batteries have been used successfully in grid scale storage as well for short duration use, and in island grid systems. Sodium-sulfur batteries are another stationary application with high efficiency. Japan has demonstrated this technology at multiple locations for six hour duration for peak shaving purposes.4 While both of these battery chemistries have been tested and deployed they are still not nearly as common as lithium-ion batteries, which currently dominate the market.
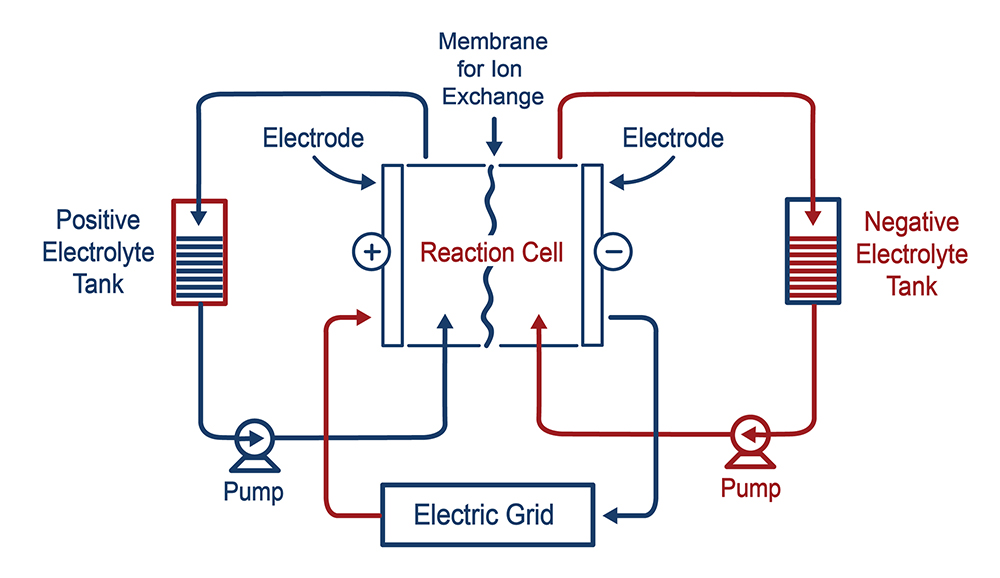
Flow Batteries
Flow batteries consist of a reversible chemical reaction, which occurs in multiple tanks. The electrodes are dissolved in electrolyte solutions stored in tanks – an anolyte tank containing an anode and a catholyte tank containing a cathode. These are pumped into cell stacks where the reversible reactions occur when the battery charges and discharges.5 There are two main flow battery systems: True flow or pure flow where all materials are stored separately from the cell and hybrid flow batteries where one or more of the active materials are stored within the cell.
Flow batteries can also vary depending on the materials used for the chemical reaction in the electrolyte tanks. Flow batteries store energy in the liquid electrolyte tanks while traditional batteries store energy in their electrode materials. Flow batteries have high energy efficiency, long life cycles, can be long duration with fast response times as well as being capable of deep discharges. However, electrolyte stability is always a concern along with potential corrosion of materials.
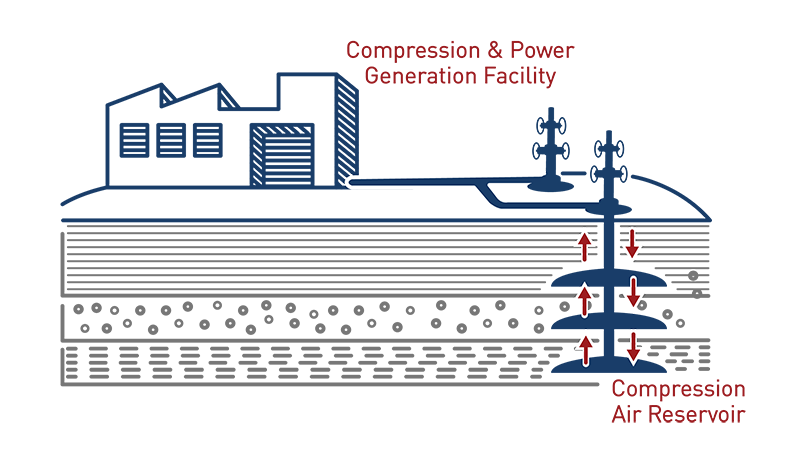
Compressed Air Energy Storage
Compressed Air Energy Storage or CAES stores energy in the form of compressed air in an underground reservoir for use at a later time. CAES is very similar to pumped hydro power in storage concepts, however, usage of the stored air is different than simply releasing water through a turbine. CAES systems release the pressurized air by heating it in order to expand it, which then turns a turbine, generating electricity.6 This is done through two systems – diabatic or adiabatic. Diabatic systems are really hybrid systems, where natural gas is used to heat the compressed air, resulting in expansion and a way for the turbine to generate electricity. Adiabatic systems do not use natural gas to reheat the air in the expansion process, rather the excess heat is stored above ground for future use when the air is meant to be expanded. Currently, only two CAES diabatic grid scale systems exist, one in Alabama and one in Germany. CAES have several benefits, but ideally they work best in balancing energy, for greater integration of renewable energy, ancillary services for the grid such as regulation, black-start, and grid stabilization.
Compressed Air Energy Storage
Compressed Air Energy Storage or CAES stores energy in the form of compressed air in an underground reservoir for use at a later time. CAES is very similar to pumped hydro power in storage concepts, however, usage of the stored air is different than simply releasing water through a turbine. CAES systems release the pressurized air by heating it in order to expand it, which then turns a turbine, generating electricity.6 This is done through two systems – diabatic or adiabatic. Diabatic systems are really hybrid systems, where natural gas is used to heat the compressed air, resulting in expansion and a way for the turbine to generate electricity. Adiabatic systems do not use natural gas to reheat the air in the expansion process, rather the excess heat is stored above ground for future use when the air is meant to be expanded. Currently, only two CAES diabatic grid scale systems exist, one in Alabama and one in Germany. CAES have several benefits, but ideally they work best in balancing energy, for greater integration of renewable energy, ancillary services for the grid such as regulation, black-start, and grid stabilization.
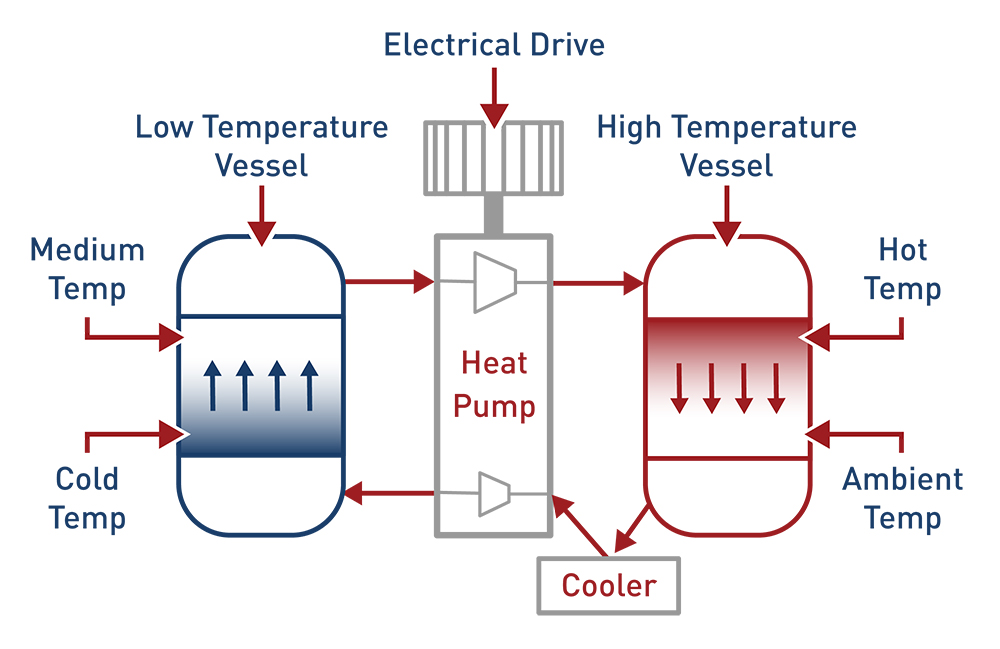
Heating and Cooling of Materials
Some promising industry experiments are focused on storing excess energy in the form of thermal energy in materials such as rocks. The temperature in these substances can be cold or hot as there is energy in both forms. Siemens is currently conducting an experiment in heating rocks with excess energy until it is needed at a later point, when it will be used to drive a steam turbine to generate electricity.7
Traditional Pumped Heat Electrical Storage compresses and expands gas through tanks filled with crushed rocks. This is usually a closed circuit where the gas is connected between a compressor and expander heating and cooling the crushed rocks as needed to store or use energy.
Hydrogen Energy Storage stores electrical energy in hydrogen through electrolysis. This means electricity is used for hydrogen production through a process called electrolysis where water molecules are split into oxygen and hydrogen ions. The oxygen is released and the hydrogen is stored in pressurized containers and can be used as a fuel to be burned in combined cycle gas fired power plants or re-electrified later through fuel cells.8
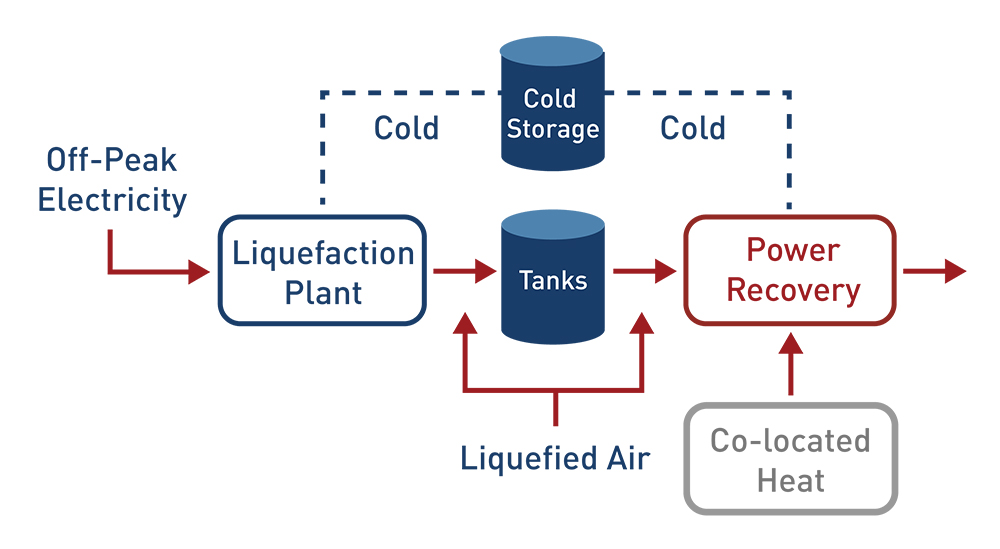
Liquid Air Energy Storage
Liquid Air Energy Storage stores energy through compressed and liquefied air. This works in three steps – charging occurs when electrical energy pulls air from the environment, cooling the air until it liquefies and is then stored in an insulated tank at low pressure until it is needed later. When the energy is needed, the liquid air is pulled from the tank and pumped to a higher pressure, where it evaporates and is heated, producing a high pressured gas that is used to turn a turbine.9
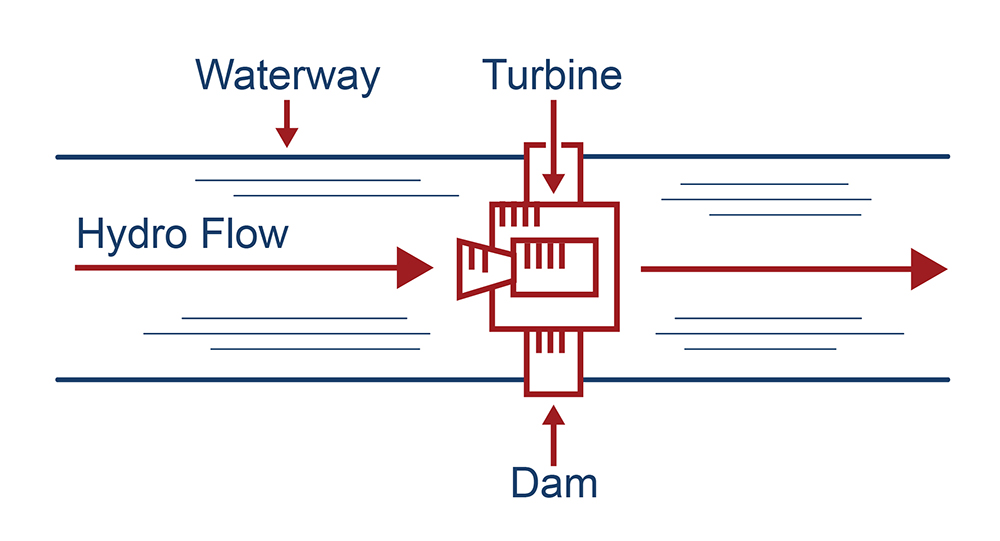
Pumped Hydropower uses gravity to turn a turbine. While this technology has been successful since the 1920s, it has been modified for additional uses such as underground pumped hydro, reservoir pumped hydro, and variable speed pumped hydro.10 In many cases, pumped hydropower is used as a form of baseload electricity generation because it is reliable and inexpensive. However, over time it has become much more complex, and can be used in various ways to help improve grid stability and even act like a “peaker plant,” used at times when peak energy demand is at its highest to help reduce consumption of natural gas in natural gas peaker plants through variable speed pump-turbines.
