Posted on January 17, 2024 by Natalie Houghtalen
Nuclear fuel burns, but not in the way you might think, like wood, where carbon bonds are broken to create heat. Instead, when the fuel burns, nuclear reactions break the atoms themselves. Nuclear energy has zero emissions and only creates a small amount of well-accounted-for waste. In fact, the amount of waste for one year of an American person’s energy use would be the size of a gummy bear, and a lifetime of energy use would fit in a soda can.
There are two main types of reactors used to generate electricity worldwide: pressurized water reactors and boiling water reactors. Both run on low-enriched uranium fuel pellets that heat water and drive a steam turbine. They were first developed in the 1950s and fall under the “light-water reactor” umbrella. Today, around 18 percent of the United States’ total electricity demand is met with only 92 reactors.
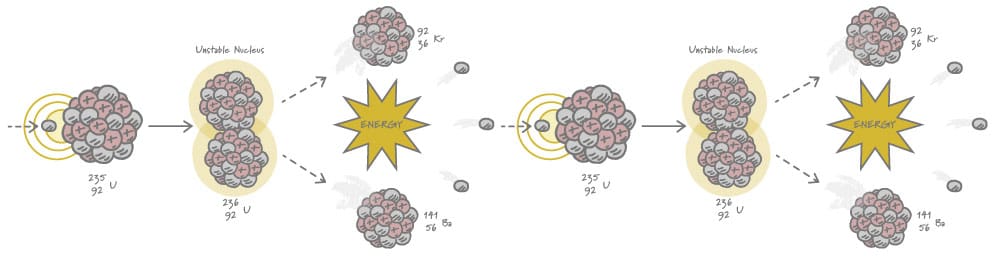
All nuclear reactors function on the same principle: a neutron hits a uranium-235 atom causing it to break apart into two big pieces and three new neutrons. This process is called nuclear fission, and uranium-235 is the only naturally occurring isotope that can do this. The two big pieces shoot out, bump into other atoms, and slow down. This process, which is essentially nuclear rug burn, creates the heat used to boil water and ultimately generate power.
Nuclear Fission Process
Nuclear interactions are all around us. In fact, 50 percent of the heat in the Earth’s core comes from radioactivity. This is an incredible, natural process. The question is, how do we harness it to create electricity?
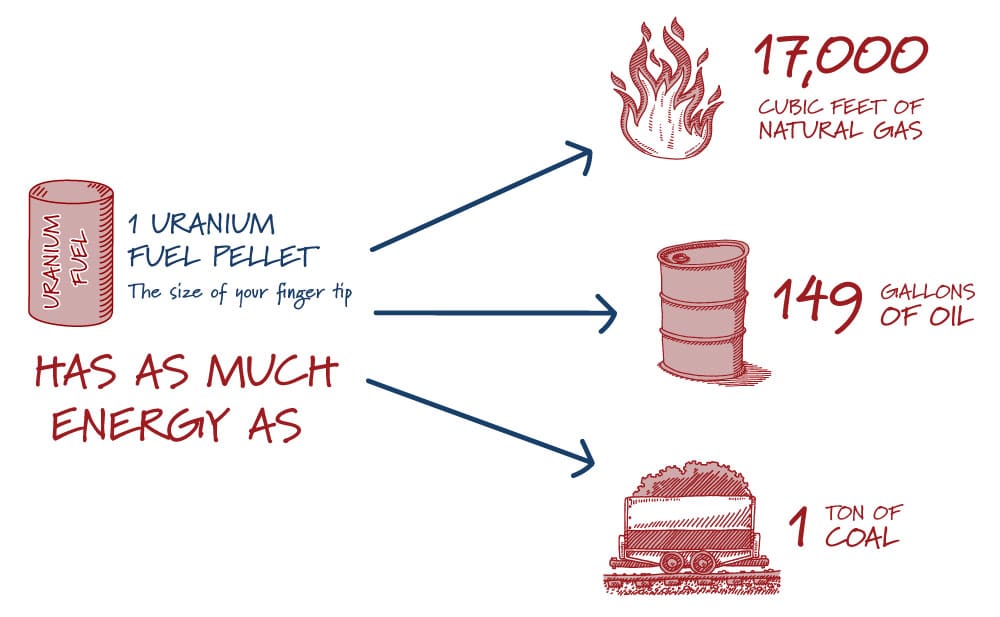
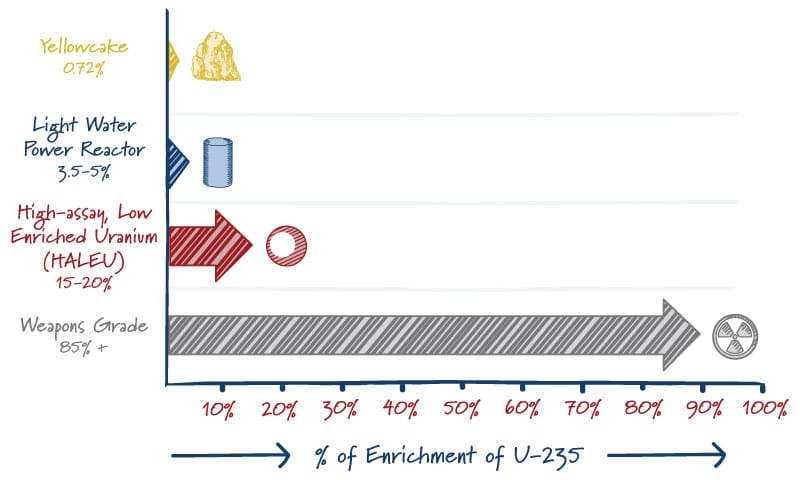
Uranium Enrichment by Product
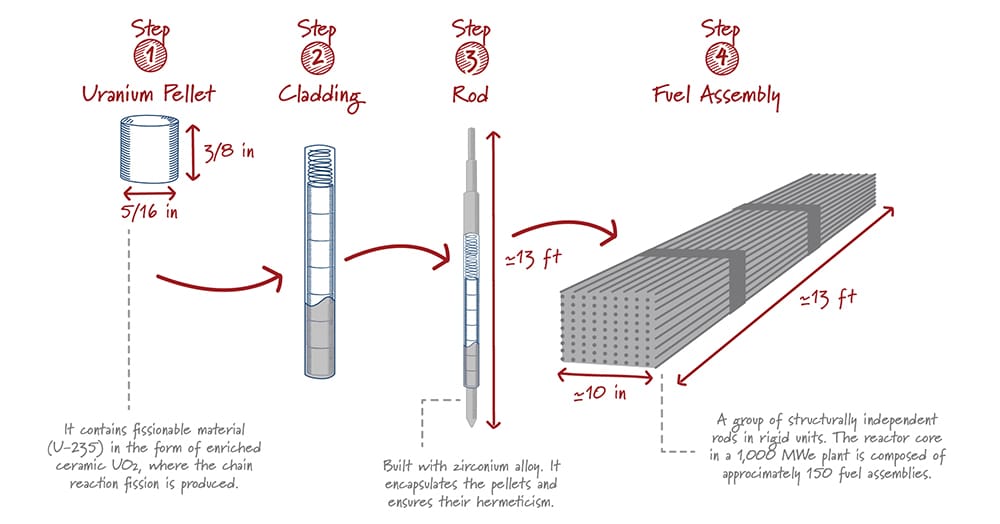
Manufacture of a Fuel Assembly